Overview of the Science Park

The Nigeria Bio Science Park, Vision 2030 Roadmap:
The The Nigeria Bio Science Park is a state-of-the-art bioscience hub located in Kano, Nigeria, dedicated to advancing Africa's biopharmaceutical and MedTech healthcare industries. It will provide cutting-edge facilities for the manufacturing and development of in-vitro diagnostics (IVDs), vaccines, and biologics. The park will consolidate, whenever possible, Nigeria’s Healthcare industry into one hub to encourage and facilitate technology transfer and sharing of facilities and knowledge. The park will include a training center (the Africa Bio Training Center - ABC) to build local and continental expertise in these fields and a Bio, MedTech, Digital-Health incubation center (the Africa BioMed Center - ABMC), to support emerging Bio, MedTech and Digital-Health African startups. The park will include as well a University-Industry joint Innovation Center (UIIC) that will help foster collaboration between Industry and Academia, accelerate innovation, support talent development and encourage entrepreneurship.
By integrating manufacturing, R&D, training, University-Industry synergy and an incubation center, the The Nigeria Bio Science Park will drive innovation, enhance healthcare, and foster economic growth in Nigeria and across Africa. The The Nigeria Bio Science Park will be established in three phases:
By integrating manufacturing, R&D, training, University-Industry synergy and an incubation center, the The Nigeria Bio Science Park will drive innovation, enhance healthcare, and foster economic growth in Nigeria and across Africa. The The Nigeria Bio Science Park will be established in three phases:
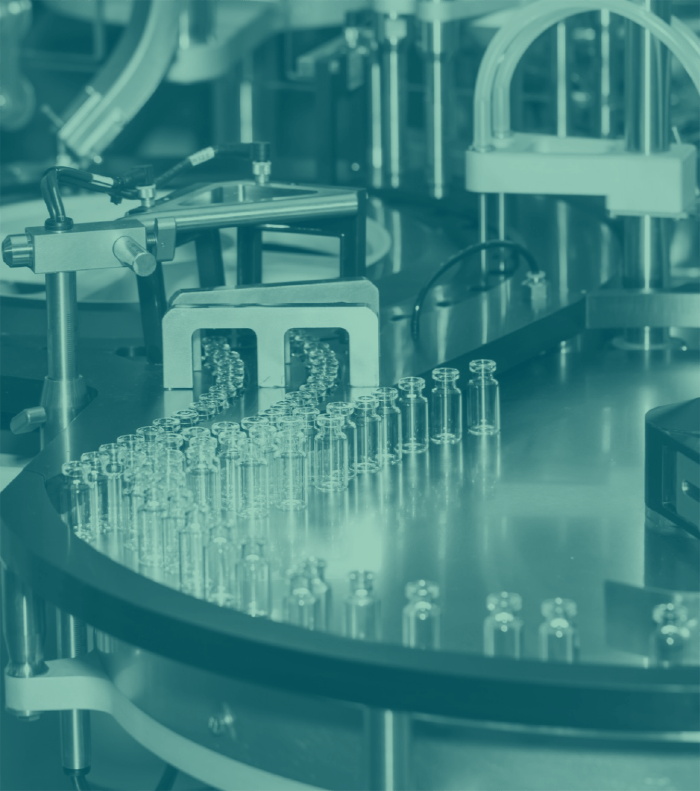
Phase 1a:
Technology Transfer of IVD Manufacturing and R&D (2024-2025)
Phase 1a focuses on establishing Plant 1 for the manufacturing and development of rapid in-vitro diagnostic (IVD) test kits. This phase includes setting up facilities for the production of diagnostic tests for diseases such as HCV, HIV, Malaria, H. pylori, Tuberculosis, HBV, and HCG. The scope involves developing and validating manufacturing lines, implementing rigorous quality control measures, and creating a robust research and development (R&D) unit. The R&D component will drive innovation in diagnostic technologies, while the manufacturing segment ensures the reliable production of high-quality test kits. Additionally, this phase will involve the construction of the Africa Bio Training Center (ABC), dedicated to training local talent in IVD technologies, vaccine production, and biologics manufacturing. The ABC will provide state-of-the-art training facilities and curricula to build a skilled workforce. This phase will also initiate the development of a University-Industry Research Center, partnering with local and African universities, including faculties of Medicine, Pharmacy, Engineering, and Bioengineering, to foster collaborative research and innovation.
Phase 1a focuses on establishing Plant 1 for the manufacturing and development of rapid in-vitro diagnostic (IVD) test kits. This phase includes setting up facilities for the production of diagnostic tests for diseases such as HCV, HIV, Malaria, H. pylori, Tuberculosis, HBV, and HCG. The scope involves developing and validating manufacturing lines, implementing rigorous quality control measures, and creating a robust research and development (R&D) unit. The R&D component will drive innovation in diagnostic technologies, while the manufacturing segment ensures the reliable production of high-quality test kits. Additionally, this phase will involve the construction of the Africa Bio Training Center (ABC), dedicated to training local talent in IVD technologies, vaccine production, and biologics manufacturing. The ABC will provide state-of-the-art training facilities and curricula to build a skilled workforce. This phase will also initiate the development of a University-Industry Research Center, partnering with local and African universities, including faculties of Medicine, Pharmacy, Engineering, and Bioengineering, to foster collaborative research and innovation.


Phase 1b:
Technology Transfer of Vaccine Fill-Finish and Biologics Manufacturing (2025-2027)
Phase 1b is dedicated to setting up Plant 2 for the fill-finish manufacturing of vaccines and biologics. This phase will commence with the fill-finish processes for vaccines such as HPV, PCV, and Hexavalent, as well as biologics like Adalimumab and Bevacizumab. The scope includes constructing sterile facilities equipped with advanced filling and packaging technology, implementing stringent quality control measures, and ensuring compliance with international regulatory standards. In parallel, this phase will advance the development of the University-Industry Research Centers, integrating local and African academic institutions to support vaccine and biologics research. The goal is to establish a reliable and efficient production line for essential vaccines and biologics, enhancing availability and treatment options across Africa. Social amenities and recreational facilities will also be introduced to support the well-being of employees and visitors.
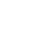
Phase 1b is dedicated to setting up Plant 2 for the fill-finish manufacturing of vaccines and biologics. This phase will commence with the fill-finish processes for vaccines such as HPV, PCV, and Hexavalent, as well as biologics like Adalimumab and Bevacizumab. The scope includes constructing sterile facilities equipped with advanced filling and packaging technology, implementing stringent quality control measures, and ensuring compliance with international regulatory standards. In parallel, this phase will advance the development of the University-Industry Research Centers, integrating local and African academic institutions to support vaccine and biologics research. The goal is to establish a reliable and efficient production line for essential vaccines and biologics, enhancing availability and treatment options across Africa. Social amenities and recreational facilities will also be introduced to support the well-being of employees and visitors.
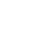

Phase 2:
Technology Transfer of Glass Vials Production and Expansion of Research Facilities (2025-2027)
Phase 2 focuses on establishing Plant 3 for the production of glass vials, both sterile and non-sterile, used in packaging pharmaceuticals, vaccines, and biologics. This phase involves setting up production lines for vial forming, manufacturing, and packaging, ensuring adherence to global standards for sterility and quality. Additionally, this phase will continue expanding the University-Industry Research Center, furthering collaborations with local and international academic and industry partners to advance research in pharmaceutical and biopharmaceutical fields. The scope also includes developing additional social and recreational areas within the park, such as walking paths, sports facilities, and community spaces, to enhance the quality of life for employees and foster a collaborative environment.
Phase 2 focuses on establishing Plant 3 for the production of glass vials, both sterile and non-sterile, used in packaging pharmaceuticals, vaccines, and biologics. This phase involves setting up production lines for vial forming, manufacturing, and packaging, ensuring adherence to global standards for sterility and quality. Additionally, this phase will continue expanding the University-Industry Research Center, furthering collaborations with local and international academic and industry partners to advance research in pharmaceutical and biopharmaceutical fields. The scope also includes developing additional social and recreational areas within the park, such as walking paths, sports facilities, and community spaces, to enhance the quality of life for employees and foster a collaborative environment.


Phase 3:
Construction of the Park’s Central Laboratories and Expansion of Social Facilities (2025-Ongoing)
Phase 3 involves constructing the Park’s Central Laboratories, crucial for comprehensive testing and analysis across various bioscience disciplines. This phase includes setting up state-of-the-art laboratories for analytical testing, stability studies, and regulatory compliance assessments. The Central Laboratories will support the manufacturing processes at Plant 1 and Plant 2, ensuring rigorous testing and validation of IVDs, vaccines, and biologics. In parallel, the development of the Africa Bio Research Institute (ABDI) will be undertaken, focusing on biopharmaceutical and MedTech incubation, to foster innovation and support emerging startups. Social amenities will also be expanded, including additional recreational areas, wellness facilities, and community engagement spaces, creating a vibrant and supportive environment for all park stakeholders.
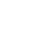
Phase 3 involves constructing the Park’s Central Laboratories, crucial for comprehensive testing and analysis across various bioscience disciplines. This phase includes setting up state-of-the-art laboratories for analytical testing, stability studies, and regulatory compliance assessments. The Central Laboratories will support the manufacturing processes at Plant 1 and Plant 2, ensuring rigorous testing and validation of IVDs, vaccines, and biologics. In parallel, the development of the Africa Bio Research Institute (ABDI) will be undertaken, focusing on biopharmaceutical and MedTech incubation, to foster innovation and support emerging startups. Social amenities will also be expanded, including additional recreational areas, wellness facilities, and community engagement spaces, creating a vibrant and supportive environment for all park stakeholders.
I The future:
By 2030, the The Nigeria Bio Science Park will be a premier bioscience hub in Africa, integrating advanced manufacturing facilities for IVDs, vaccines, and biologics with cutting-edge research and development centers. It will house the Africa Bio Training Center (ABC) for workforce development, a University-Industry Innovation Center for collaborative R&D, and the Africa Bio Research Institute (ABDI) to support startups. This integrated ecosystem will bolster Nigeria and Africa’s capabilities in healthcare and biopharmaceuticals, reduce dependency on imports and build self-reliance, and drive economic growth through innovation, skilled talent development, and enhanced access to critical medical products
Facilities and Infrastructure
The industrial park covers an area of 9.8 hectares, including vaccine production workshop, IVD production workshop, QC laboratory, automated warehouse, administrative building, research and development building, and supporting infrastructure, responsible for the research and production of biological products.
The industrial park is equipped with tap water filtration system, chilled water system, compressed air system, purified water system, HVAC, dehumidification system, and sewage treatment system to ensure that the production environment meets production requirements. The site and workshop will be designed by top global design institutes, and top suppliers will be selected to ensure that the production workshop meets GMP requirements.
The industrial park is equipped with tap water filtration system, chilled water system, compressed air system, purified water system, HVAC, dehumidification system, and sewage treatment system to ensure that the production environment meets production requirements. The site and workshop will be designed by top global design institutes, and top suppliers will be selected to ensure that the production workshop meets GMP requirements.
Strategic Localization
Commitment to a Sustainable Operating Model: The The Nigeria Bio Science Park, including all activities it conducts within the Park is committed to environmental protection, sustainability, and social responsibility, integrating these principles into its construction and management.
- The park will be designed with eco-friendly infrastructure, utilizing advanced waste management systems to minimize environmental impact.
- Water conservation practices and green spaces will enhance the site's ecological balance.
- The park will also adhere to stringent Environmental, Social, and Governance (ESG) standards, ensuring ethical operations and transparent governance.
- Community engagement and social responsibility initiatives are central to its mission, providing local employment opportunities, supporting education,and promoting health and well-being.
- By prioritizing sustainability and social responsibility, the Nigeria Bio Science Park aims to create a positive and lasting impact on both the environment and the communities it serves.








